Title: Water Testing- Temperature and pHOverviewAfter completing the unit on Color, Odor, and Foam, students should be familiar with their stream site and comfortable making visual observations. They may then begin making physical measurements of the water, beginning with two basic parameters: temperature and pH. Both of these parameters can be important in and of themselves (high temperatures can kill fish, high or low pH may indicate pollution). They are also important because of the effect they have on other parameters that we will measure, such as dissolved oxygen or ammonia toxicity. For this reason we always measure temperature and pH in the field, even if these are not the focus of our study. Grades: High School Kansas Grade Level Expectations
Kansas Environmental Education Standard 1: Learners demonstrate an understanding that the earth is system.
Environmental Education
Standard 4, Benchmark 1 Standard 4: Learners develop the abilities necessary to conduct scientific inquiries.
9-12 Benchmark 2: Learners demonstrate scientific inquiry skills.
MaterialsIntroduction to Unit on Water Testing – Temperature and pHWhen first arriving at a stream site, a scientist makes and records observations of the weather, the land surrounding the stream, and the condition of the water. If you completed the previous lesson in this section on Color, Odor and Foam, you already have a set of observations recorded in your field notebook. After observing the site, the next step is to do scientific tests on the water. The tests you perform will vary depending on what you want to know about the water. For example, if you want to know what effect the surrounding corn fields are having on the stream, you may test for nutrients. If you want to know if the water is safe to swim in, you would want to test the bacteria levels. No matter what you want to know, you should almost always begin by testing temperature and pH. This is because temperature and pH are very simple tests that can tell you a lot about the condition of the stream. Also, many other features you may test for are dependent on temperature or pH, so knowing these values will help you figure out other things about the water, such as the ammonia toxicity level. As you learn about temperature and pH in this unit, be sure to remember your observations and make predictions about the results you will find. There will be more information in each section about making predictions based on your observations. Title: Water Testing- TemperatureOverview: Presentation to StudentsKnowing the temperature of the water is very important. Ammonia toxicity, dissolved oxygen, and many other tests are directly related to the temperature of the water, so if you forget to measure the water temperature, you may not be able to calculate other parameters later. The temperature of the water will be loosely related to the temperature of the air. Of course, surface water cools in the winter and warms in the summer along with air temperature. However, water changes temperature more slowly than air, so it is possible to have warm air temperatures and cold water or vice-versa, especially in the spring and fall. Before you measure temperature, refer back to your observations in your field notebook and make a quick prediction about the water temperature. Many different factors determine the temperature of the water. The air temperature, especially over the last few days, can help warm or cool the water. A stream with a very healthy riparian zone (trees and grasses on the bank) is more shaded than if there is no riparian area (crops or urban development right up to the streambank). You can predict that shaded water will have a cooler temperature than a stream in full sun. If the stream is spring-fed, the cold groundwater will cool down the temperature of the stream. If it has rained recently, think about the land use around the stream. Rain water running over streets and parking lots picks up a lot of heat, especially in the summer. When that water reaches a stream or river, it heats up the temperature of the water. In some places the storm runoff heats up the water so much that it makes it impossible for native fish, plants, and other species to live there anymore. This has been referred to as temperature pollution or thermal pollution. Discharge from industrial sources can also warm up stream water. This is considered point-source pollution and is more strictly regulated than non-point source pollution as described above. Kansas Department of Health and Environment regulations state, “Discharge from artificial origin shall not elevate the temperature of the water above 32C (90F) and not raise the temperature more than 3C above natural conditions.” Remember that even a small change in temperature can make an aquatic environment inhospitable for many native species. After you make your predictions, you are ready to perform the actual test. Tests for temperature should always be performed right at the site, because of course once you take a sample out of the stream, it will begin warming up or cooling down depending on the air temperature. Because of this, other tests that are directly related to temperature (dissolved oxygen, ammonia, etc.) should also be performed on-site if possible. Grades: High School Objective: Determine the temperature of water at a sampling site. MaterialsThermometer, ideally one designed for rugged use (non-mercury, encased in hard plastic or other material to reduce the chance of breakage). Be sure to tie a string or cord around your thermometer so that you can hold on to it easily without losing it to the current. Ordering information can be found here. MethodSecure the string or cord around your wrist or a sturdy object on the bank, so that you do not lose your thermometer in the stream. Place the thermometer approximately where you will be collecting your sample. You don’t want to measure temperature right at the edge, or too deep in the middle. The temperature profile of the stream will change depending on where you are measuring, so be sure to get a measurement that is representative of the sample you will be taking. Allow the thermometer to adjust to the water temperature for a few minutes. Record your reading in your field notebook. Be sure to record whether the reading is in Fahrenheit (F) or Centigrade (C ). If you need to convert from one unit to another, use the formulas below: [°C] = ([°F] - 32) × 5/9 [°F] = [°C] × 9/5 + 32 Refer to the chart below, and record a rating for your stream. Temperature Rating
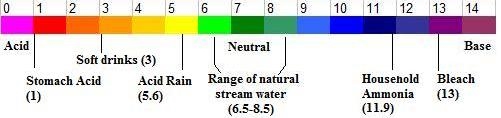
InterpretationThe temperature of the water, along with your observations of surrounding land use, can tell you quite a bit about the health of the stream. An area with a warm water temperature that has no trees to shade the water and lots of cement on the surrounding banks is most likely warmer than it should be naturally. Aquatic life such as fish, plants, and other living organisms are probably physically stressed, or may no longer be living in that area at all. Title: Water Testing- pHOverview: Presentation to StudentsThe pH of water is a measurement of how acidic or basic it is. It is measured on a scale of 1 to 14. On this scale, 7.0 is neutral (neither acidic nor basic), 0 is the most acidic, and 14 is the most basic. You may also hear people refer to chemicals as acid or alkaline - alkaline is another way to say "basic." This scale can be confusing, because most people grow up hearing about the corrosive properties of acids, but not much about bases. Very basic substances are just as dangerous as very acidic substances. An illustration of the pH scale is shown below. The pH of pure water (water which has little to no ions or other substances dissolved in it) is neutral, pH 7. Water in streams and lakes often has many things affecting it, including the surrounding rocks and soil, land use, chemicals from runoff, waste from aquatic animals, and decaying organic material. These things can make water in streams, lakes, and rivers slightly acidic (down to pH 6.5) or slightly basic (up to pH 8.5). Look around your site. Do you see any yellowish, chalky rocks in or around the water? This is limestone, a basic (alkaline) rock that is very common in Kansas. Many old houses and University buildings on campuses in Kansas are made out of limestone. As water dissolves limestone, it becomes basic. Because of this, don't be surprised if the water at your stream site tests slightly basic. Industrial facilities have to be very careful about the pH of the water they discharge (called effluent). If it is too acidic or too basic, it can greatly harm the aquatic life of the stream. Also, air pollution from industrial facilities and machinery emissions causes acid rain in some regions of the country. Acid rain can reduce the pH of surface water to dangerous levels. Fortunately, acid rain hasn't been a major problem in Kansas. The toxicity of ammonia is directly related to the pH of the water. The same amount of ammonia is much more toxic if the pH of water is 8.5, compared with water that is pH 6.5. If you are doing nutrient measurements on your stream site, pH is an important number to know. It is best to perform the pH test at the site, or as soon as possible after collecting the sample. Grades: High School ObjectiveDetermine the pH of water at a sampling site. MaterialspH test strips (ordering information can be found here) MethodsTake out the number of strips you'll need for your water samples and replace the cap. Dip a test strip into your water sample and remove immediately. Don't shake the excess water from the test strip! Hold the strip level for 15 seconds. Compare the pH test pad to the color chart on the bottle, and record your results in your field notebook. If the color appears to be between two color blocks on the chart, estimate the result (probably as halfway between the two colors - for example if the color of your test strip appears to be between 6 and 7, you may record the result as 6.5). Refer to the chart below, and record a rating in your field notebook.
InterpretationGenerally, if pH is within the normal range for surface water (6.5 to 8.5), it is ok. There may be other problems affecting the water, but the pH is within acceptable range. If the pH is outside of this range, there is almost definitely something negatively affecting the water. *For an advanced description of pH in natural surface water, please see the Advanced pH page. | ||||||||||||||||||||
What's in the Water? >